Pharmacare has introduced a solid presence in the Palestinian market throughout the years and has maintained a prestigious reputation among its counterparts. Pharmacare has been reputed as one of the best companies in Palestine in compliance with various national and international criteria. It was the first and only Palestinian company to have acquired and maintained the EU c-GMP Certificate and subsequently started exporting to EU countries.
Milestones

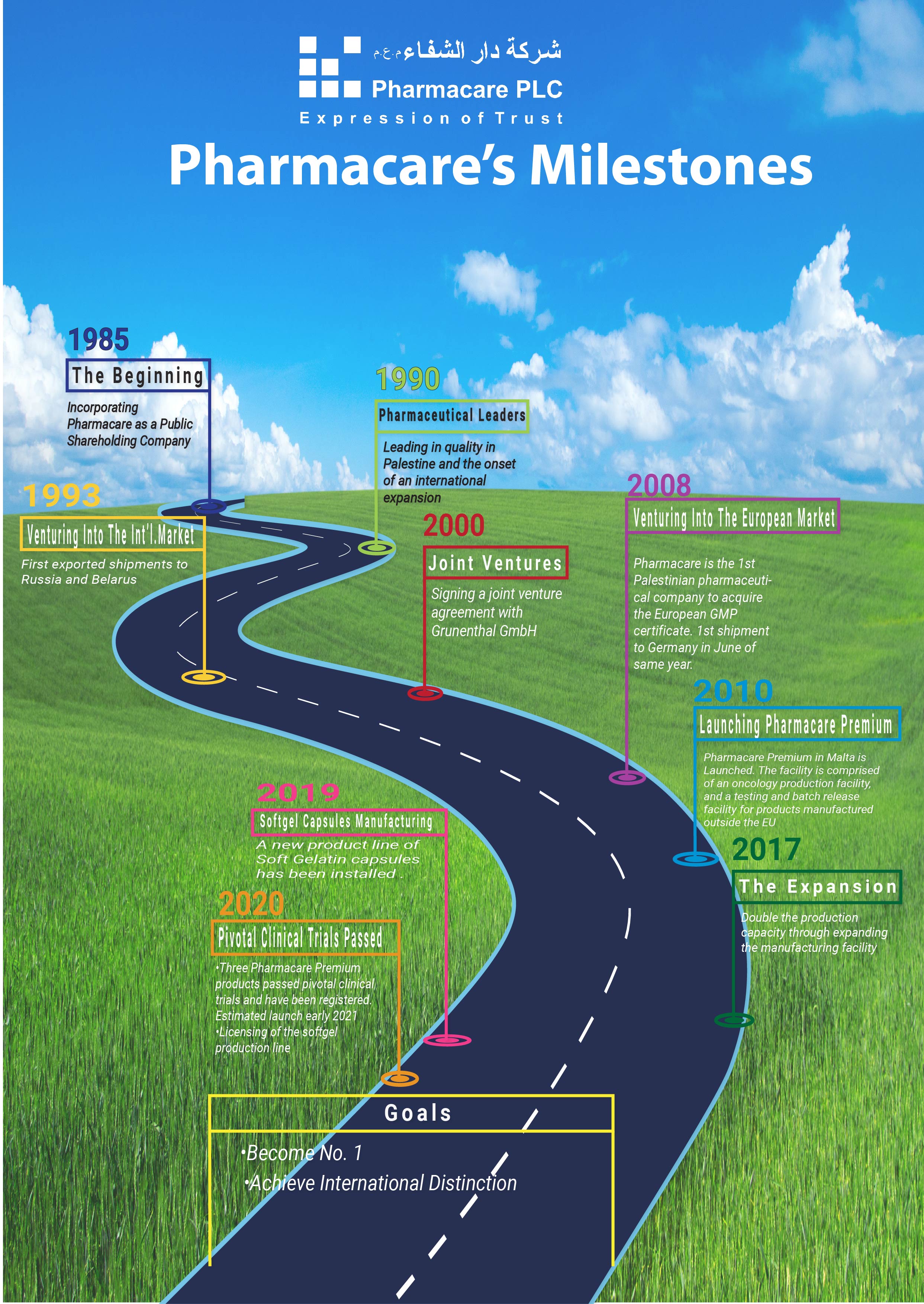
Pharmacare was established in 1985 and has hit several key milestones since then:
- 1985-86 - foundation Charter and building of the manufacturing facility: The first Palestinian company to take into consideration GMP guidelines - The British “Orange” Guide.
- In November 1986 Amoxicare®, the first product produced by Pharmacare was introduced to the local market.
- 1987- First international inspection by French experts. Pharmacare declared in the forefront of Palestinian companies in GMP guideline implementation.
- 1987 Established the first computer department as a separate entity in the company, ushering in the IT era into the Palestinian industry.
- 1988- First computer network in the country was established at Pharmacare.
- 1990- A strategic decision to expand internationally, with continuous local expansion in products and volumes.
- 1990- First American inspection by a former FDA inspector. PCP obtained an excellent assessment.
- 1993 - First shipment to Russia and Belarus. Pharmacare becomes the first company to export pharmaceuticals from Palestine. Currently Pharmacare is ranked no.12 in the Belarusian market.
- 1999 - 2000 - Pharmacare signed a joint venture agreement with Grunenthal GmbH of Aachen, Germany giving it 22% of Pharmacare.
- 2000 - Pharmacare inaugurated its state of the art multimillion-dollar manufacturing facility rendering it c-GMP compliant. Thus, Pharmacare is able to adhere to the continuously changing c-GMP guidelines, particularly implementing the zoning system which requires complete separation and independent access into production, packaging, labs and stores.
- 2000 - In the same year, Pharmacare received ISO 9001 and ISO 14000 certifications.
- 2000-2007- Pharmacare expanded its operations by introducing new therapeutic lines, diversifying the existing ones, and increasing its sales.
- November 2007- Pharmacare signed a supply agreement with Grunenthal GmbH to export Tramadol capsules to Germany. Grunenthal will pack and sell the product in Germany and other EU countries.
- December 2007- Pharmacare acquired the Palestinian Good Manufacturing Practices (GMP) after passing the official audit conducted by Ministry of Health and external experts.
- 2008 (The Great Success) - Pharmacare is the 1st Palestinian pharmaceutical company to acquire the European GMP certificate. This great success allowed Pharmacare to enter the European market, which enables further expansion of the company into an international establishment and benefiting the Palestinian economy by increasing the contribution to Palestinian GNP.
- June 2008- Pharmacare celebrated the first shipment of its medicine to Germany.
- 2009 –Malta was identified as a strategic location for expansion. After a long study of market needs, especially for the EU and MENA region it was decided to have a multi-faceted (multi-functional) facility. The major part is an oncology production facility, while the other part is a testing and batch release facility for products manufactured outside to EU and released to the European market.
- 2010 – Pharmacare Premium in Malta is officially inaugurated in the presence of the Maltese President, Mr. George Abela and other dignitaries from Malta, Palestine and Germany as the first Palestinian facility in EU.
- 2010- Large amounts of products were exported to the EU. By the end of 2010, about 21% of Pharmacare’s production was exported to European countries.
- January 2011- Pharmacare successfully renewed its European c-GMP certificate for the coming 3 years. This great success reflects the ability of Pharmacare in maintaining excellence.
- February 2011- Pharmacare Premium acquired the EU GMP approval, and now as a certified facility, it is permitted to start operating as a batch release site.
- 2012 – Pharmacare PLC acquires the Latin American and Brazilian ‘’ANVISA-Granted’’ GMP.
- 2013 – Renewal of EU-GMP
- 2014 Renewal of the Palestinian GMP.
- 2014 –2015 A new major expansion of Pharmacare’s facilities was initiated following approval of a long-term loan by the French Government. The ribbon cutting ceremony was held in September 2015 in the presence of the then French Minister of Economy and current president, Emanuel Macron. This expansion allows Pharmacare to introduce new production lines and Pharmaceutical forms and preparations. Explore More
- 2016 – Palestinian GMP Renewal; Belarus GMP Renewal; EU-GMP Renewal
- 2016 – 2017 – Building professional relationships with the Polish Pol-Pharma in arrangement of both under-license and marketing authorisation holding agreements permitting the manufacturing of Pol-Pharma products in Palestine and the distribution of the Pharmacare products within a variety of European countries, respectively.
- 2016 - 2017 -HikmaCare (HC): Pharmacare entered into a joint-venture with Hikma Pharmaceuticals manufacturing under-licence strategic Hikma Pharmaceuticals’ products, which will be distributed within the West Bank and the Gaza Strip. Explore More
- 2017 – Renewal of the ISO 9001 and 14001 according to the 2015 guidelines and regulations
- 2017 –Stage 1 of the new major expansion of Pharmacare’s facilities was officially inaugurated in September in the presence of the French Minister of Economy; Mr. Bruno Le Maire and Minister of Palestinian National Economy; Ms. Abeer Odeh. Explore More
- 2017 – 2018 - The establishment of the Soft Gel Facility functioning in the manufacturing of soft gel capsule medicinal products.
- 2018 - The Iraqi Pharmaceutical Industry (Baghdad) of which Pharmacare owns 10%, re-inaugurated its state-of-the art production facility, received GMP certificate from the Iraqi Ministry of Health. Explore More
- November 2019 - EU GMP certificate renewed for 5th consecutive time, proving Pharmacare’s commitment complete compliance with good manufacturing practices that ensures the continued production of quality products and validating its motto “Expression of Trust”.
- April 2019 – Renewal of the ISO 14001 according to the 2015 guidelines and regulations.
-
May 2020 - Renewal of the ISO 9001 according to the 2015 guidelines and regulations.
-
July 2020 –Finished Installing a 500 KW solar panel grid to harness the power of the sun and reduce its reliance on fossil fuel, as part of Pharmacare’s concern for lowering its carbon footprint.
-
2020- Softgel product line is licensed by the MoH and is ready to start production of medications. With its introduction, Pharmacare will be the first Palestinian pharmaceutical company to locally manufacture softgel pharmaceuticals, and will be the beginning of the launch of a new product line of softgel products.
-
2020- Three new Pharmacare Premium’s oncology products pass pivotal clinical trials and then registered. The products are expected to be launched in early 2021.
-
October 2020 – Beitunia Municipality Plan to upgrade it road infrastructure: Road renovation of the area adjacent to Pharmacare’s campus is completed of which Pharmacare contributed 60% of the costs. This is part of Pharmacare’s public-private sector partnership, and Pharmacare’s commitment to shouldering its fair share in its corporate social responsibility.
-
November 2020- Approval of the new strategic plan Pharmacare 2025.
A Closer look into a bright history:
Key Historical Stops
3-D design Plan

The coming era marks the introduction of Pharmacare’s expanded manufacturing facility on-setting in 2018. The facility comprises eight floors, three undergrounds and an interconnecting tunnel. 6.2 m have been expended, of which a 2.5 m long-term loan has been, kindly, endowed by the AFD. This expansion is anticipated to double Pharmacare’s production capacity and should trace a trademark in future documentation of the company’s history.


A Mark of Excellence
GMP Palestinian Certificate 2017
ISO 9001 - 14001
Brazilian Certificate
Pharmacare on The News
The Pharmacare Album