The Pharmacare Potential
Pharmacare is a leading interdisciplinary corporation bringing together a diversity of experienced professionals and promising new graduates, assuring proper training for quality recruitment and rewarding outcomes. Currently, a team of 330 employees, 70% are graduates of higher education institutions holding bachelor's, master's and PhD degrees in chemistry, pharmacy, biology, computer science and business, work at Pharmacare PLC in Palestine.
Pharmacare is composed of highly qualified professionals, from pharmacists and researchers to technical specialists and business experts, contributing together to a well established organization functioning towards quality production. Hence, Pharmacare presents a solid foundation within the production of generic pharmaceuticals, extract products, medical devices and a variety of other services in collaboration with unique partners. Together, Pharmacare maintains a standard of quality and well reflects its emblem denoting an “Expression of Trust”
Established as a public shareholding company in 1986, Pharmacare is located in a small environment-friendly industrial zone in Beitunia within the Ramallah district, 15 km north of Jerusalem. Starting with a humble setup and upgrading few years later towards a state of the art c-GMP certified facility in the beginning of 2000, the company was ushered into an era of continued growth. With the new expansion embarked upon in 2015, the facility currently comprises eight floors, three of which are underground. A tunnel spanning throughout the lowest underground interconnects the company’s various facilities.
Pharmacare’s iconic quality manufacturing hit a zenith towards 2007/ 2008 on pursuing the EU c-GMP (European Current Good Manufacturing Practice) certificate following a series of successes on passing several international inspections and audits throughout the past couple of decades. In 2009, it founded the holding company, Pharmacare Europe (PCE) as a 100% owned subsidiary of PCP.
The PLC Umbrella
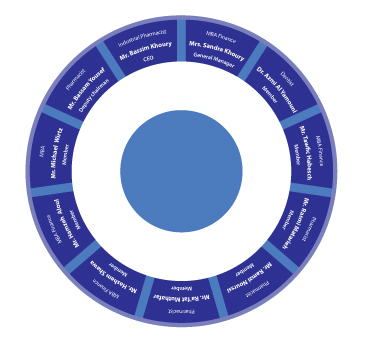 The Pharmacare Umbrella comprises the package presenting the key sites and activities performed within the Pharmacare Group. From Generic Pharmaceuticals comprising veterinary products, beta-lactam manufactures and the recruitment of soft-gel for drug delivery systems, to our Oncology facility in Malta, Pharmacare leads a crucial role in meeting the local needs of pharmaceuticals on the domestic scale and responding to cancer-based demands on a global level. It’s the joint department of Research and Technical Development that serve the skeleton upon which Pharmacare predicates in venturing towards the far-fetched goals.
The Pharmacare Umbrella comprises the package presenting the key sites and activities performed within the Pharmacare Group. From Generic Pharmaceuticals comprising veterinary products, beta-lactam manufactures and the recruitment of soft-gel for drug delivery systems, to our Oncology facility in Malta, Pharmacare leads a crucial role in meeting the local needs of pharmaceuticals on the domestic scale and responding to cancer-based demands on a global level. It’s the joint department of Research and Technical Development that serve the skeleton upon which Pharmacare predicates in venturing towards the far-fetched goals.
Pharmacare PLC has initiated a series of businesses in relation to natural supplements (Nutricare), as well as internationally manufactured cosmeceuticals and medical devices, which are anticipated to set record beginning 2019.

-
Generic Pharmaceuticals
 Pharmacare PLC Palestine (PCP) is a leading pharmaceutical company within the State of Palestine. PCP specialises in the domestic development, production and marketing of generic and proprietary branded pharmaceuticals, and the local distribution of global pharmaceutical products within the Palestinian field.
Pharmacare PLC Palestine (PCP) is a leading pharmaceutical company within the State of Palestine. PCP specialises in the domestic development, production and marketing of generic and proprietary branded pharmaceuticals, and the local distribution of global pharmaceutical products within the Palestinian field.
Throughout the past twenty-five years since its foundation, PCP has succeeded to demonstrate a model role in the Palestinian economy and a consistently growing profile over its home market. With over 100 products and 200 employees, PCP supplies premium affordable medicines to the local population. PCP, further, reflects incessantly increasing returns over its shareholders and continues to develop as an international business currently covering wide-range geographical locations worldwide.
-
The Beta-Lactam Facility
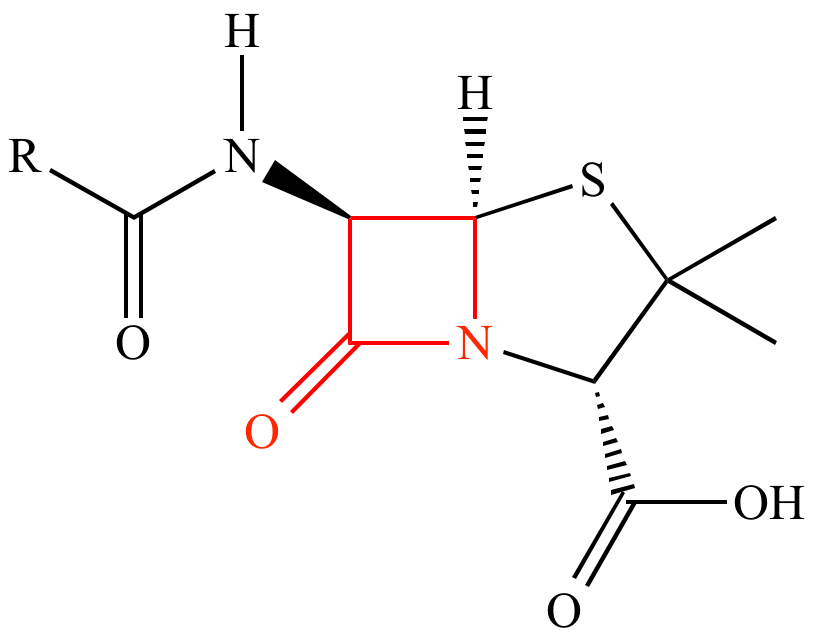
Pharmacare’s High Potency Beta-Lactam facility is located within an environment-friendly industrial zone in Beitunia, Ramallah, Ein Arik Street. Though situated within the immediate vicinity of the main Conventional Factory, the R&D, and T&D departments, it completely separated and is at a distance to insure no cross contamination. It is recruited for the manufacturing of Penicillin and Cephalosporin as tablets, capsules, and suspensions.
Working hours are well considered to safeguard unwarranted risks of confusion, cross-contamination, and accidents.
The facility presents an area of 1,250 meters squared and builds on an overall of 1 acre, thus presenting three floors, of which the warehouses and storage units constitute the first and the second.
The facility is certified by both Belarus and the Palestinian GMPs, thereby permitting the exporting of the manufactured products to many countries, such as Belarus, Kazakhstan, Georgia, Azerbaijan, and Armenia. There are more than 30 products manufactured within this facility, all of which are registered by the Palestinian Ministry of Health.
-
The Veterinary Facility - Al-Nur
Pharmacare’s Al-Nur facility for veterinary products is located within an environment-friendly industrial zone in Beitunia, Ramallah. It functions in the manufacturing of oral powders and solutions and sterile liquid and powder injection vials giving rise to a generous range of veterinary items.
Working hours are well considered to safeguard unwarranted risks of confusion, cross-contamination, and accidents.
The facility is certified by the Palestinian Ministry of Health for the production of veterinary sterile products, as well as veterinary liquid solutions and oral powders. There are more than 50 products manufactured within this facility, all of which are registered by the Palestinian Ministry of Health.
-
Soft Gel Capsule Line

Pharmacare’s Soft Gelatin Capsules production line is located within an environment-friendly industrial zone on the Ein Arik Street in Beitunia, Ramallah. It functions in the manufacturing of medicines filled in soft gelatin capsules.
Working hours are well considered to safeguard unwarranted risks of confusion, cross-contamination, and accidents.
This facility line will be used for the production of soft gelatin capsule medicines and the production of granular medicines in sachets.
The Soft Gelatin Capsules’ Line Department was designed and established according to stringent cGMP regulations and equipped with fully automated equipment.
Oncology
Pharmacare Premium (PCM), a manufacturing company dedicated to oncology was founded in 2005 in Malta, 65% of its shares are owned by Pharmacare Europe (PCE). Based in Hal-Far Industrial Zone, Malta, PCM marks an elite subsidiary of the Pharmacare Group, thus managing an EU certified manufacturing, testing & Batch Release Centre for pharmaceutical products entering the EU.
Development

Research and Development – R&D
The Research and Development, R&D, a department at Pharmacare PLC is a 200-meter square area headed by Dr. Sana' Mousa. The department is divided into research and analytical laboratories; doing both Chemical and Microbiology Research.
Our current R&D novelty pipeline concerns working on the purification of herbal products; the work conducted is anticipated to reflect promising results within 2019/ 2020.
Technical Development – T&D
The Technical Development / Product Development department at Pharmacare PLC is an 800-meter square area headed by Mr.Ayman Qaddumi. The department is divided into:
- A Formulation Laboratory (Product Development) is the place where trials are carried out. This pilot-scale manufacturing area is equipped with a set of all required machinery needed for pilot trials.
- An Analytical Laboratory (Method Development), where we develop the analytical methods for the analysis of raw material and finished pharmaceutical products.
- A Stability Laboratory, where all stability studies and shelf-life determinations required for the registration of new products and re-registration of old products are performed. This section is equipped with a variety of chemical lab instrumentations, such as HPLC, Dissolution Testers, Moisture Content Analysers, Ultraviolet Spectrophotometers ... etc.
Our current TD pipeline highlights special focus on:
- Formulations of new generic products, which are comparable in efficiency, safety, and quality to those of the brand products. An annual plan for those products is prepared by specialized committees within the Pharmacare organization.
- Modification and improvement of existing products in regard to quality and efficacy with respect to physical properties and chemical characteristics as well as to overcome particular drawbacks.